A Review of Clinical Studies in Elderly Populations
Share this webinar on your network:
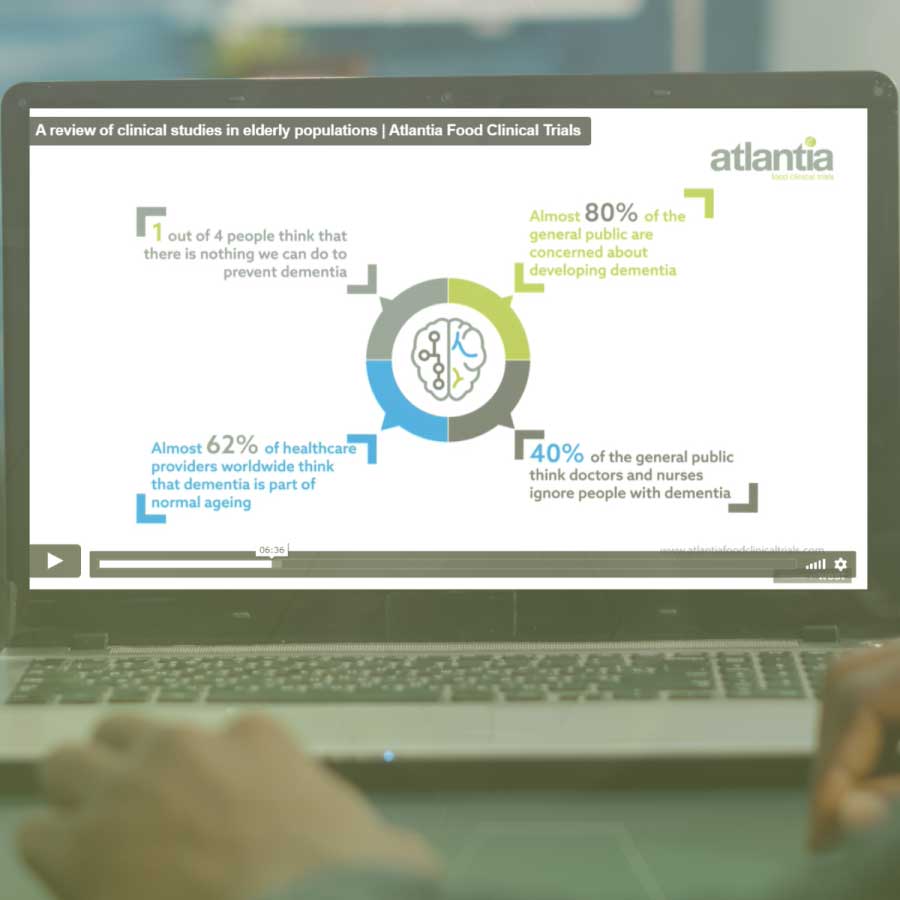
In 2018, people aged 65 years or over worldwide, outnumbered children under age five for the first time in history. By 2050, an estimated 1.5 billion people will be aged 65 or older. The healthy ageing industry is expected to become highly competitive and profitable. In functional food industries, differentiation is key. The first step of which is to prove the health benefits potentially associated with products. Human clinical studies are considered the gold standard method for the scientific substantiation of claims by the main health institutions and a powerful marketing and sales tool. However, there are some challenges and considerations when designing and conducting clinical trials involving elderly participants. Join us to unveil the secrets of human clinical studies within an ageing population.
Duration: 25 mins
Webinar
Speakers

Barry Skillington
Barry helped build the brand and customer base to where it is today.
Chief Commercial Officer
Atlantia Clinical Trials

Emily Goodbody
Over 10 years of experience in a multitude of clinical trials in different health areas.
Senior Operations Manager
Atlantia Clinical Trials
