Dedicated End-To-End Clinical Trial Experts
From study design to reporting, our in-house experts deliver everything you need to successfully launch your product.
Get everything you need to run your study from start to finish with Atlantia's end-to-end trials.
Eliminate outsourcing costs, obtain ultra-clean data, and boost your ROI with Atlantia's in-house clinics and specialized recruitment, operations, data, science, and biostatistics teams.
Fixed Pricing Upfront
3x Faster than Other CRO's
Customized Trial Design
Fixed Pricing Upfront
3x Faster than Other CRO's
Customized Trial Design
200+ Trials Completed
The Atlantia Proof Playbook
Starting with your protocol right through to data delivery, Atlantia's Proof Playbook support your entire clinical journey, delivering the proof your product needs for commercial success.
- Flexible Design & Protocol
- Tailored Study Plans
- Ethics/IRB Submission
- Regulation - EFSA, FD, MDR, EMA
- In-house Recruitment Team
- Database Segmentation
- Marketing Campaigns
- Automated Pre-Screening
- Dedicated Sponsor Success Team
- Conduct Visits
- Data & Biological Sample Collection
- Independent Monitoring
- Trial Master File
- Biological Sample Prep/Analysis
- Data Review
- Biostatistical Analysis
- Study Reports & Publication
- Single Point of Contact
- Own USA & EU Facilities
- Easier Communication
- Faster Decisions
- No Outsourcing Costs
- Dedicated In-house Teams
The Atlantia Proof Playbook
Starting with your protocol right through to data delivery, Atlantia's Proof Playbook supports your entire clinical journey, delivering the proof your product needs for commercial success.
1. Design & Comply
Flexible Designs & Protocol
Tailored Study Plans
Ethics / IRB Submission
Regulation - EFSA, FDA, MDR, EMA
2. Recruit & Screen
In-house Recruitment Team
Database Segmentation
Marketing Campaigns
Automated Pre-Screening
3. Conduct Study
Sponsor Success Team
Conduct Visits
Data & Sample Collection
Independent Monitoring
Trial Master File
4. Analyse & Report
Biological Sample Prep/Analysis
Data Review
Biostatistical Analysis
Study Reports & Publication
5. Value Multiplier
Single Point of Contact
Own USA & EU facilities
Easier Comms, Faster Decisions
No Outsourcing Costs
Dedicated In-House Team
Our Populations
We recruit diverse participants across various demographics to ensure your study reaches its full potential with accurate and representative results.
Hybrid
Trials
- Internal Science/Ops
- International Partner Sites
- Own clinics in USA & EU
- Medical Network
- Virtual PI
Onsite
Trials
- Internal Science/Ops
- International Partner Sites
- Own Clinics in USA & EU
- Medical Network
- Expert PI
Remote
Trials
- Sponsor Project Team
- Custom Apps/Wearables
- Real-Time Data Access
- Telehealth integration
- Virtual PI
PK / Bioavailability
- In-house Biostatisticians
- Analysis per Population Set
- Cmac, Tmac, AUC, Half-Life
- PK/PD Modelling
Our Clinical Trial Models
Whether on-site, hybrid, or remote, we tailor each model to enhance participant engagement and simplify your research.
Our Clinical Trial Models
Whether on-site, hybrid, or remote, we tailor each model to enhance participant engagement and simplify your research.
Hybrid
Trials
- Internal Science/Ops
- International Partner Sites
- Own clinics in USA & EU
- Medical Network
- Virtual PI
Onsite
Trials
- Internal Science/Ops
- International Partner Sites
- Own Clinics in USA & EU
- Medical Network
- Expert PI
Remote
Trials
- Sponsor Project Team
- Custom Apps/Wearables
- Real-Time Data Access
- Telehealth integration
- Virtual PI
PK / Bioavailability
- In-house Biostatisticians
- Analysis per Population Set
- Cmac, Tmac, AUC, Half-Life
- PK/PD Modelling
International Trial Sites
Our multinational clinic sites offer access to diverse populations across America and Europe. With partner sites in key locations, we deliver flexibility and reach for robust data collection and timely trial success.
Ideally located in Chicago, Illinois, our facilities include:
- Central Phlebotomy Lab
- IP Prep Room
- Access Controlled -80° -20° Refrigerated/Ambient/Biosample & IP/File Storage
- Sample Preparation Lab
Ideally situated in Cork, Ireland, our facilities include:
- Central Phlebotomy Lab
- IP Prep Room
- Access Controlled -80° -20° Refrigerated/Ambient/Biosample & IP/File Storage
- Sample Preparation Lab
- 50+ Partner Sites across both the USA and Europe.
- Broad array of specialities
- Advanced facilities and expertise
- Seamless collaboration and integration
- Global Medical Network
Tech Powered, Human Led
We use the latest technology for efficiency and safeguard it with expert human oversight to mitigate the risk of bias.
Our experienced biostatisticians catch what AI algorithms might miss and tease out nuanced insights for exceptional accuracy.
Our Technology
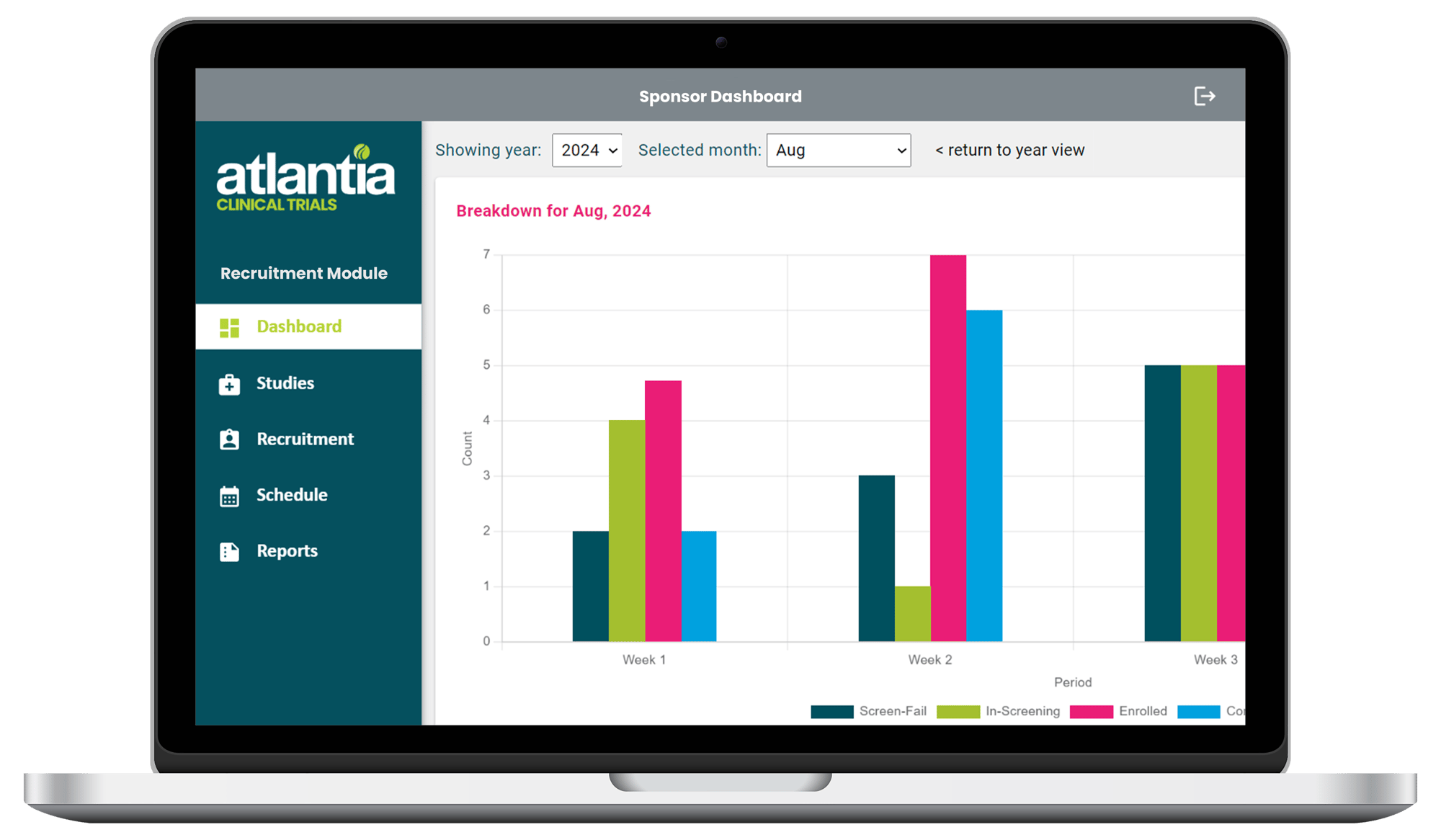
Track Your Study's Recruitment Progress in Real Time
Get all the recruitment updates you need, whenever you need them with Atlantia's Sponsor Dashboard.
Recruitment Funnel
Enrolment Rate
Screening Breakdown
Screen Fail Reasons
Custom Built Participant Apps
Our custom, study-specific apps include validated questionnaires like IPAC, SF-36, GSRS, EPIC FFQ, PSQI, PSS, POMS, and the Bristol Stool Scale, along with support for sensors and wearables, delivering real time data capture and deeper insights into participant health.
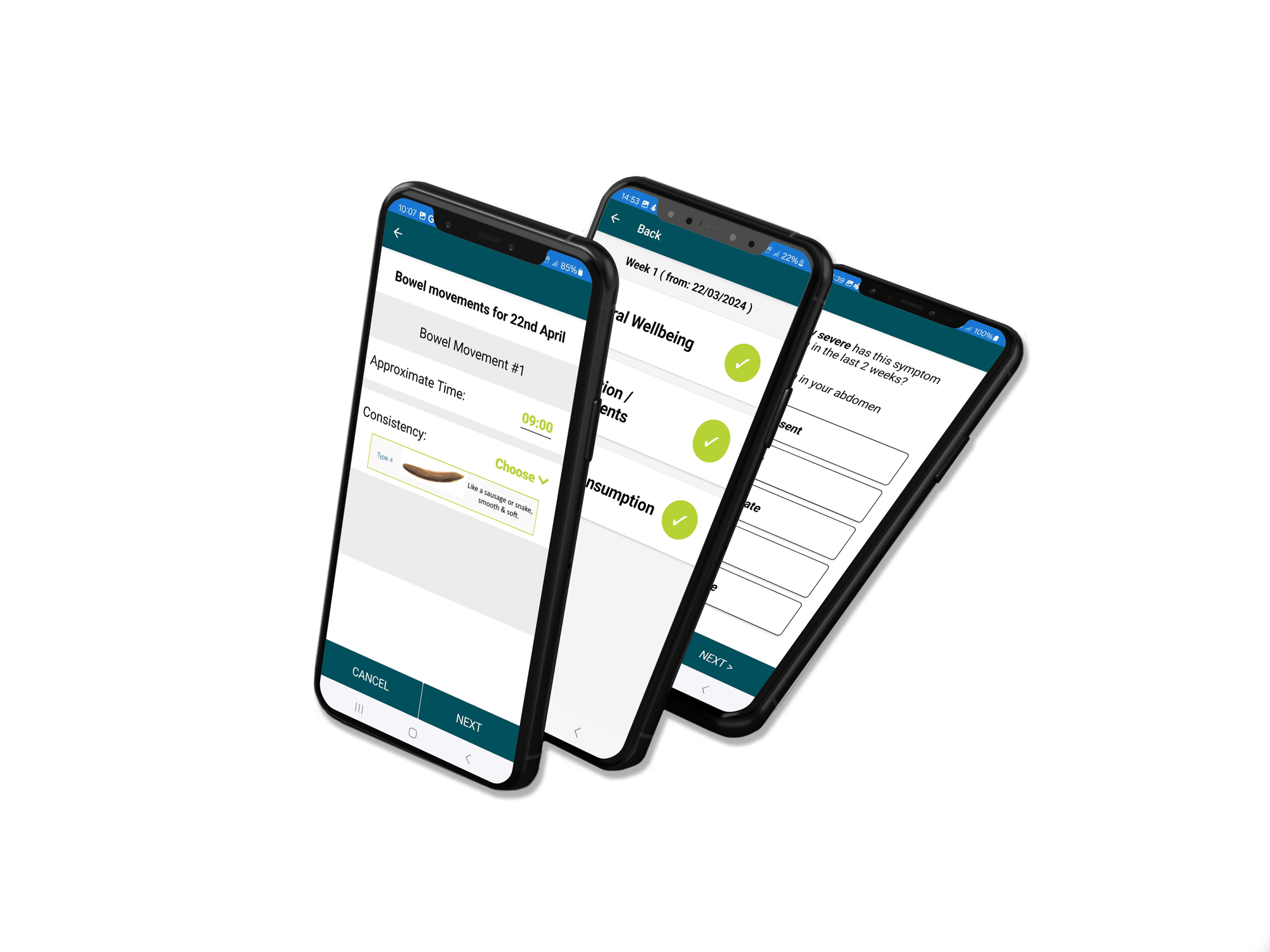

Data Collection & Analysis Technology
We have a range of technology across multiple health areas to successfully execute your study.
16S rRNA Sequencing
Antera 3D
BIA
Blood Pressure Monitors
CANTAB
CDR System
CGM
COMPASS
Corneometer
CT Scan
Cutometer
DEXA Scanner
Dynamometer
e-Diaries
Flow Medicated Dilation
Fluorescence Activated Cell Sorting
Gastric Scintigraphy
Galvonic Skin Response
Lipidomics
Mexameter
MRI
Next Generation Sequencing
Nutritics Software
Oximeter
PCR
PET Scan
Radiopaque Markers
Shotgun Sequencing
Spirometer
Tewameter
Video Camera Endoscopy
Visiometer
VO2 Max
Wearable Devices
Ready to Lead the Market with Science?
Kickstart your clinical program today. Get in touch with our sales team to discuss your project.
