Beauty from Within
Build consumer trust and cement your brand's market leadership, fueling growth and loyalty.
Atlantia Clinical Trials excels in the Beauty from Within sector, offering science-backed solutions for substantiating your ingestible beauty claims. Our method identifies your product's unique challenges—from improving skin health to ensuring safety—and designs efficient, effective trials to solidify your claims.


Skin Microbiome
Meet regulations and boost your marketing approach by highlighting you dedication to science-backed beauty solutions.
As a leader in Skin Microbiome products, Atlantia offers unparalleled research and expert collaborations to differentiate your products in the market. We foster partnerships with top researchers to harness the full potential of your products. Our trials highlight your product's interaction with the skin's microbiome, providing robust evidence of efficacy and safety.
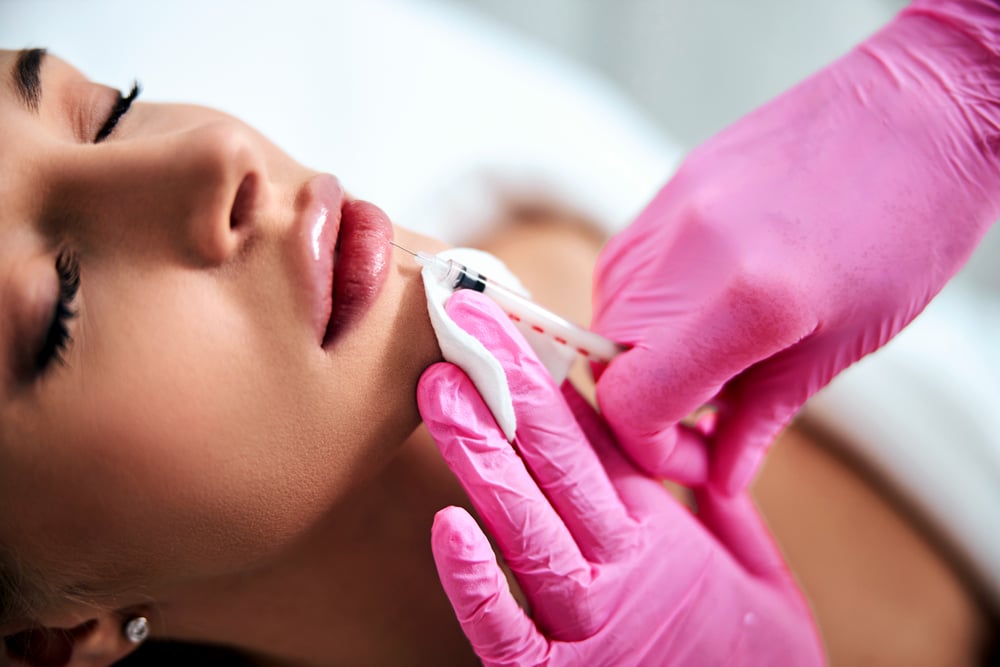
Atopic Skin Conditions
Showcase your product's effectiveness to build trust and establish a competitive advantage.
For products targeting Atopic Skin Conditions, proving efficacy and safety is paramount. Atlantia leverages a vast database of diverse volunteers, including those with chronic conditions like dermatitis, rosacea, and psoriasis, to conduct tailored trials. Our approach ensures products meet scientific standards and fulfill the needs of affected individuals, providing strong evidence to support your claims and distinguish your brand.

SOLUTIONS
The Atlantia Difference
Expert Team
Our team of expert clinical research professionals is well-versed in the ICH-GCP standard and brings a wealth of knowledge in medical, data quality science, and regulatory compliance.
Owned Facilities
We own and operate two cutting-edge clinical facilities, one located in Cork, Ireland, and the other in Chicago, USA. Our facilities feature private consultation rooms, central phlebotomy/PK areas, and specialized equipment such as DXA and bioimpedance machines.
Medical Network
We collaborate with private hospital groups and medical organizations that offer specialized medical supervision and patient referrals for specific patient-focused trials. Whether serving as a CRO, recruitment site, or both, we help you optimize your resources and time effectively.
Hybrid Trials
Strike the perfect balance between rigor and flexibility with our hybrid trial model. Tailored to suit the specific requirements of your study, our trials are crafted to enhance efficiency, speed, and participant involvement while keeping costs in check.
FAQ
How does Atlantia ensure regulatory compliance for "Beauty from Within" products?
Atlantia meticulously navigates the complex regulatory landscape for "Beauty from Within" products by aligning study designs with EFSA and FDA guidelines, ensuring that all health claims are substantiated and compliant. Our deep understanding of both food supplement and cosmetics regulations ensures your products meet global standards.
What approach does Atlantia use to validate the efficacy of microbiome beauty products?
Our validation process involves microbiological analysis and clinical trials to demonstrate how our sponsors' products positively impact the skins microbiome. This scientific rigor supports the development of compelling, evidence-based marketing claims that resonate with consumers and comply with regulatory standards.
How can Atlantia help in marketing products designed for atopic skin conditions?
Atlantia aids sponsors by conducing clinical studies that prove their products' effectiveness in managing or alleviating atopic skin conditions that all marketing claims are accurate and supported by clinical evidence. This approach not only builds consumer trust but also adheres to the stringent regulations governing products for specific skin conditions.
We use gold standard clinical measurements to validate and verify your data from both quantitative and qualitative clinical outcomes. We can test a host of endpoints from blood, stool, urine, saliva and other outputs. We work with a range of validated tools to assess and quantify patient reported outcomes across a vast array of measurement parameters. Data can be collected directly from patients or through validated tools to report on outcomes.
What makes Atlantia's study design for cosmetic products stand out?
Atlantia's study designs stand out dur to our focus on rigorous scientific methodologies, tailored to each product's unique claims and market goals. Our affiliation with the APC Microbiome Institute enriches our capability to conduct advanced microbiome research, making our clinical trials particularly suited for substantiating claims about microbiome and skin health products.
Start Your
Clinical Trial
Page Cards
These cards are page specific, you can delete this text if it is not required.
Operating Internationally

Chicago, USA
+1 312 535 9440
142 E Ontario St #1200, Chicago, IL 60611

Cork, Ireland
+353 21 430 7442
Heron House, Blackpool Retail Park, Cork T23 R50R
