The study designing process is essential in clinical trials. A lot of consideration is needed on the type of study that is needed for your desirable outcomes.
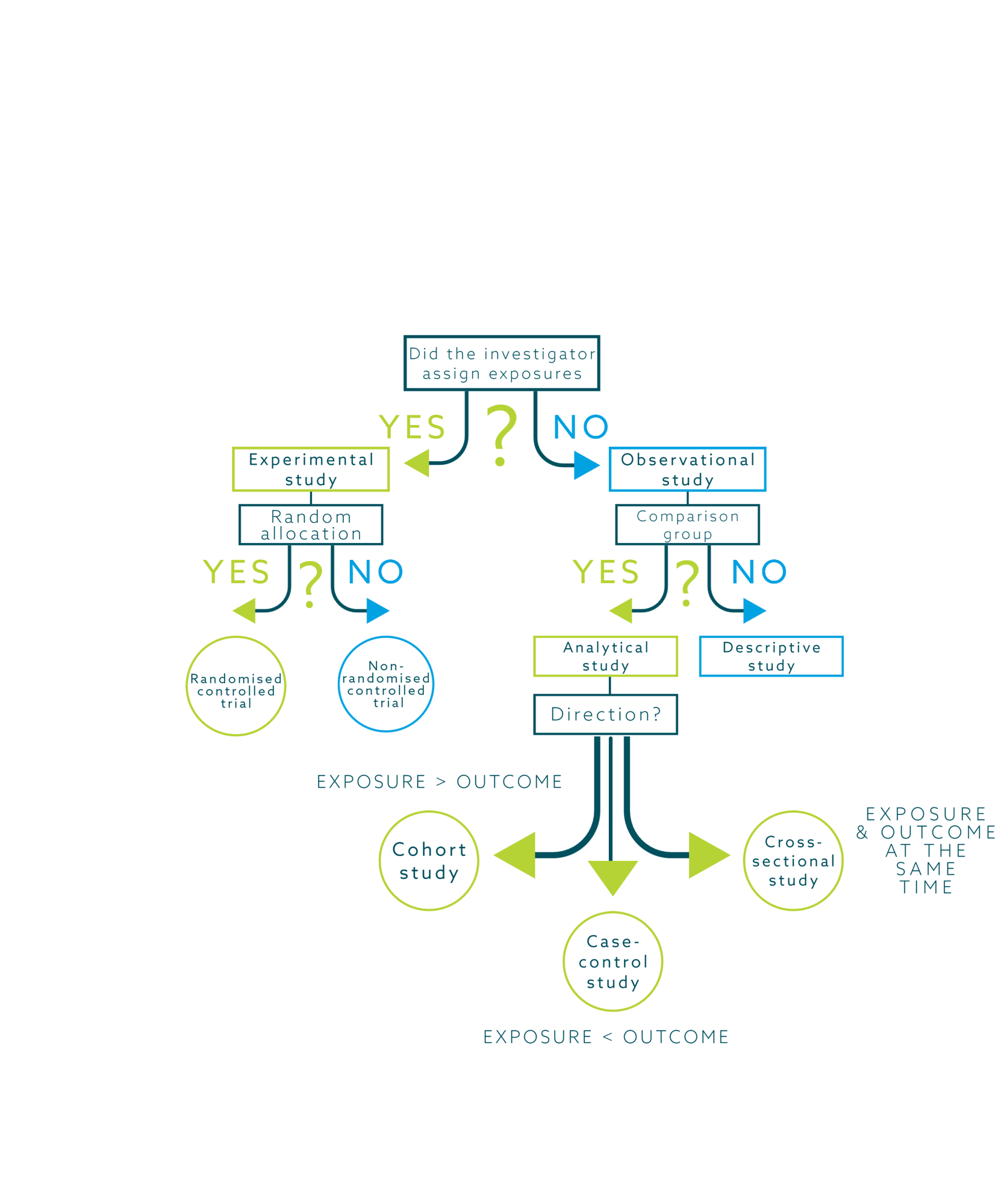
The following method describes the process involved in determining the correct design type for your future clinical trial. This includes determining the appropriate research strategy, writing justification and protocol, calculating sample sizes, deciding on criteria for subject selection and choosing the correct comparison groups. Strategically, the studies could be RCT or non-RCT: Cohort, Case-control, Cross-sectional, Observational, and Quasi-experimental. In this article, an overview will be provided on the listed study types. This will enable key innovators in coming to an inclusive decision on the appropriate study design for their desired study outcomes.
What study design should you go for?
Randomised controlled trials
Randomised controlled trials (RCTs) are the gold standard in clinical research. Dating back to 1923 RCTs involve randomly assigning participants to two or more groups (an experimental group and a control group) and comparing the outcomes of the groups to determine the effectiveness of a treatment or intervention. The results that are analyzed are used to assess the effectiveness of the intervention, which is the extent to which a treatment, procedure, or service does patients more good than harm. Although the first idea of randomization was introduced in 1923, the first randomized control trial was carried out on streptomycin in 1946.
Atlantia Clinical Trials often performs this design type. For instance, see attached a RCT study the Atlantia team conducted in the past- ‘A randomised, double-blind, parallel, placebo-controlled study to investigate the efficacy of Lactobacillus paracasei N1115 in gut development of young children’. Access the study now. In this study, infants and young children between the ages of 6 months and 3 years were administered with a probiotic consisting of LP N1115 strain (n=30) or a placebo supplement (n=30) over an 8 week intervention period.
Observational studies
Another type are observational studies: these studies observe and collect data on participants without intervening or manipulating any variables. These studies can be either prospective or retrospective. The 3 types of observational studies are: case control observational study, cohort observational study and cross sectional observational study. See examples below.
Cohort studies
Cohort studies: follow a group of people (a cohort) over time to observe the development of a disease or condition. Cohort studies can be either prospective (where the cohort is identified at the beginning of the study) or retrospective (where the cohort is identified from existing data). An example of a recent cohort study conducted is a 2020 prospective cohort study. This study Inpidual and combined associations between cardiorespiratory fitness and grip strength with common mental disorders: a prospective cohort study in the UK Biobank |BMC Medicine | Full Text (biomedcentral.com)) found an association between lower physical fitness and depression. The researchers in this study found that people with depression at baseline were more likely to experience depressive symptoms several years later if they had lower fitness levels compared with those with higher fitness.
Case-control studies
Case-control studies: compare a group of people with a disease or condition (the cases) to a group of people without the disease or condition (the controls) to identify potential risk factors for the disease or condition. The advantages of this study design over others are that they are relatively quick to perform, economical, and easy to design and implement.
Cross sectional studies
Whereas Cross sectional studies: involve collecting data on a group of people at a single point in time to determine the prevalence of a disease or condition or to explore relationships between variables.
Quasi-experimental studies
Quasi-experimental studies: involve manipulating a variable but do not involve randomisation of participants to different groups. This particular study type is thus useful in settings where randomisation is not feasible or ethical. This method is useful for studying interventions that cannot be manipulated, such as policies or environmental changes.
Similarly to the decision on RCTs & non RCT trials, choosing the appropriate/ correct comparison group is also a strategic decision: Parallel vs Cross-over studies.
Parallel clinical trials
Parallel clinical trial: participants are randomly assigned into two or more groups, each receiving a different treat mentor intervention. The groups are then observed and compared over a specified period to determine each treatment's effectiveness and safety. Parallel trials are commonly used when the treatments being compared are not expected to interact with each other or when it is not feasible to switch participants from one treatment to another. See an example of a randomised, double blind, parallel, placebo-controlled study to investigate the efficacy .The participants were randomly assigned into different treatment groups; one given the rice NPN or placebo (maltodextrin), with 75% (n=30) of subjects receiving the treatment and 25% (n=10) the placebo. Subjects were given one dose of their given product each morning and one dose each evening for the study duration of 4 weeks.
Cross-over clinical trials
Cross-over clinical trial: participants receive multiple treatments or interventions in a predetermined sequence. Each participant serves as their control, as they receive both treatments, and the effects of each treatment are compared within the same inpidual. Cross-over trials are useful when the treatments being studied are expected to have similar effects, or when there is a concern about individual variability in response to treatments. Cross-over trials can also help reduce the number of participants needed for a study. See an example of a recent study conducted by Atlantia. The study designed was a randomised, double blind, placebo-controlled, cross over trial.
See a flowchart of the decisions that have to be made when it comes to study design.
Conclusion
All types of trials can yield valuable scientific evidence when used appropriately to test various hypotheses or to observe various food or drug effects in humans. Each clinical trial type requires specific clinical trial methods and data analysis. Randomised Clinical Trials (RCTs) can be designed to test for “superiority” (one is better than the other), “non-inferiority” (one is not worse than the other) or “equivalence”(one cannot be differentiated from the other) among other study designs.
Randomised Human Clinical Trials (RCT’s) are considered the gold standard of evidence for the scientific substantiation of claims by many regulatory bodies such as EFSA & FDA.
RCT’s add medical knowledge & provide effectiveness of interventions for disease prevention. However, optimal trial design does not always require a randomized, double-blind, placebo-controlled trial. As discussed in this chapter, many types of clinical trials can yield scientifically valid results.
Want to learn more about Clinical trial design and conduct? Follow the link
/Images/64df4e04fbe9e6a17c6cdc32_Charles%20Kakilla.png?width=290&name=64df4e04fbe9e6a17c6cdc32_Charles%20Kakilla.png)

