Atlantia completed a clinical trial on behalf of the sponsors DSM Nutritional Products. The results from this study were published by the Journal of Nutrition. The background research needed for initiating this clinical study start has been explored in last week’s piece - Gut-heart axis opportunity revealed in. Keep reading to learn more about the study conduct, results revealed and the future plans.
Designing clinical trials for maximum efficacy
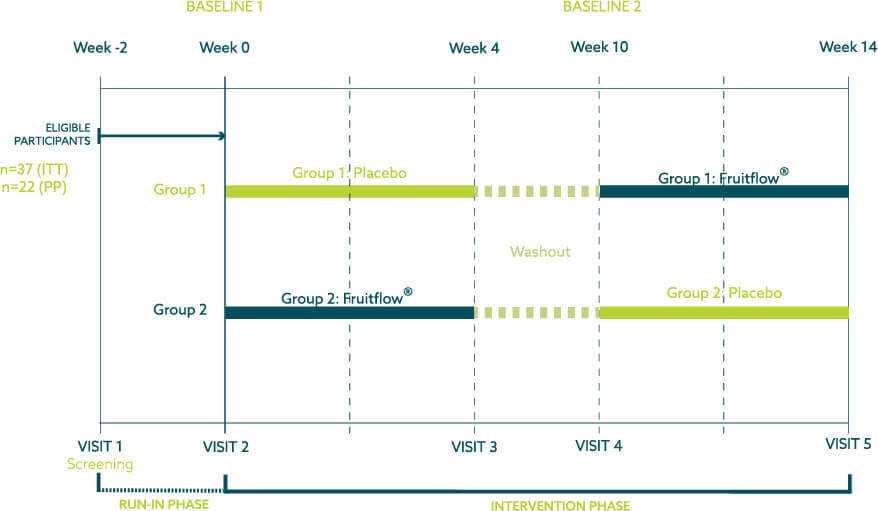
A randomized double-blind, placebo-controlled crossover study was designed and conducted by the Atlantia team in order to assess whether Fruitflow® can be used as a dietary supplement solution targeting the gut-heart axis. This study population consisted of 37 healthy, overweight, and obese adults (BMI 28-35 kg/m2), aged between 35-65 years. The study consisted of 5 visits where recruited participants attended Atlantia’s clinic site in Blackpool, Cork. A subgroup of 9 participants completed an egg challenge to determine the effect of the intervention on postprandial plasma TMAO concentrations. A standardized menu, designed to deliver a low choline diet for the 24 hours before the study visits. Learn more about our clinical trial design and clinical trial conduct offering.
Microbiome sequencing
Once the study conduct was completed, microbiome analysis was carried out by the team led by Dr. Claesson at Seqbiome Ltd, Atlantia’s strategic partner.
“ Seqbiome carried out microbiome analysis of the 16s rRNA amplicon sequence data from the collected stool samples using the state-of-the-art bioinformatics methods, followed by interpretation and production of publication ready figures and tables”.
Further analysis was required on the clinical outcomes. Atlantia’s data team led by Gillian DunnGalvin conducted this analysis.
As noted by Dr. DunnGalvin: “Atlantia provided an end to end service from protocol development to support in publication writing. The statistical team completed the analysis of the clinical outcomes of urine TMAO and plasma TMAO, LPS, hsCRP and tolerability”.
Cardiovascular CRO research findings
Results were revealed for the first time in the Journal of Nutrition. Fruitflow® was found to significantly decrease fasting blood and urine TMAO when compared to baseline and plasma LPS, which is a marker of intestinal permeability and low-grade inflammation. When compared to the placebo group, this was significantly different for urine TMAO. Dr. Steinert shares that “Fruitflow® seems to modulate the gut microbiota in a way that resulted in a lowering of urine and plasma TMAO. Moreover, we found that Fruitflow® reduced plasma lipopolysaccharides (LPS), a gut microbiota-derived factor that has been suggested to drive onset and progression of chronic inflammation related diseases such as obesity, type 2 diabetes or non-alcoholic fatty liver disease (NAFLD)”. Significant changes in microbial beta diversity were also highlighted. In addition, the microbiome composition changed significantly in the Fruitflow® product group. Lower levels of Bacteroides, Ruminococccus and Hungatella were found, which are known for the involvement in TMA/TMAO metabolism.
A conclusion can be gained at the end of this trial, as polyphenol rich extracts such as Fruitflow® can modulate the gut microbiota conferring the host a cardiovascular health benefit.
Cardiovascular and cognitive research opportunities
This study outcome reveals an opportunity for further research. Based on the results in the past study with TMAO being found as a biomarker for cardiovascular disease, it displays as a positive indicator for further research. Dr Claesson stated that “findings in this study provide support to the association between higher levels of TMAO and fecal microbiota. In this study, we identified potential TMA-producing bacteria that could be used as a prognostic biomarker or as a therapeutic target in patients with several cardiovascular conditions”.
Dr. Steinert elaborated on the potential of novel dietary solutions targeting TMAO: “thinking further this can open the way to develop novel dietary solution for cardiometabolic benefits but also benefits along the gut-brain axis. Indeed, latest suggests a role that TMAO levels may also be relevant for monitoring (age-related) inflammation and cognitive dysfunction”.
This study leads the way for significant opportunity for further research.
Dr Steinert shares that “It is exciting to see a beneficial role of Fruitflow® also on the gut microbiome. We will be developing dietary supplement solutions targeting the gut heart & gut brain axis”.
Atlantia was delighted to take on the task of completing this trial efficiently, on budget on time. Our clinical research team appreciated the acknowledgement by the sponsors of the trial on the publication.
Would you like to download this paper? Follow this link to gain access to the publication. In addition, you can visit our publications page to discover some of our most recent nutrition research!


